Neanderthal News - 2020 Part III. Health. Theirs and ours...
- The major genetic risk factor for severe Covid-19 is inherited from Neanderthals
- Neanderthal DNA highlights complexity of COVID risk factors
- Neanderthals may have had heightened sensitivity to pain
- Neanderthals were as good at tolerating smoke-related toxins as Early modern humans: study
- Neanderthal thumbs were better adapted to holding tools with handles: study
- See also:

The major genetic risk factor for severe Covid-19 is inherited from Neanderthals
Modern humans and Neanderthals could be forgiven for having other issues on their minds when they interbred in the stone age. But according to researchers, those ancient couplings laid a grim foundation for deaths around the world today.
Scientists have claimed that a strand of DNA that triples the risk of developing severe Covid-19 was passed on from Neanderthals to modern humans. The genetic endowment, a legacy from more than 50,000 years ago, has left about 16% of Europeans and half of south Asians today carrying these genes.
The origins of the risk genes came to light when scientists in Sweden and Germany compared the DNA of very sick Covid-19 patients with that from Neanderthals and their mysterious sister group, the Denisovans. The stretch of DNA that makes patients more likely to fall seriously ill closely matched that collected from a Neanderthal in Croatia.“I almost fell off my chair because the segment of DNA was exactly the same as in the Neanderthal genome,” Hugo Zeberg, an assistant professor at the Karolinska Institute in Stockholm, told the Guardian.
Zeberg and his co-author, Svante Pääbo, director of the Max Planck Institute of Evolutionary Anthropology in Leipzig, suspect the Neanderthal genes have persisted in modern humans because they were once beneficial, perhaps helping to fight off other infections. Only now – when faced with a new infection – has their downside been exposed.
It is unclear how the genes may worsen Covid-19, but one gene plays a role in the immune response and another has been linked to the mechanism the virus uses to invade human cells.
“We are trying to pinpoint which gene is the key player, or if there are several key players, but the honest answer is that we don’t know which are critical in Covid-19,” Zeberg said.
According to the study, published in Nature, the cluster of genes on chromosome three are most commonly found in Bangladesh, where 63% of the population carry at least one copy of the DNA sequence.
“The genes in this region may well have protected the Neanderthals against some other infectious diseases that are not around today. And now, when we are faced with the novel coronavirus these Neanderthal genes have these tragic consequences,” Pääbo said.
The researcher, who led the international team that first deciphered the Neanderthal genome in 2010, said his “rough estimate” was that about 100,000 “additional” people have died so far in the current pandemic due to the genetic contribution from Neanderthals.
Beyond the Covid-19 risk genes, the Neanderthals have bequeathed other genes to modern humans. Some increase sensitivity to pain, while others reduce the risk of miscarriages.
“Some are beneficial and some are detrimental,” Zeberg said. “This has been a double-edged sword.”
But Mark Maslin, a professor at UCL and author of the book The Cradle of Humanity, cautioned that the work risked oversimplifying the causes and impact of the pandemic.
“Covid-19 is a complex disease, the severity of which has been linked to age, gender, ethnicity, obesity, health, virus load among other things,” he said. “This paper links genes inherited from Neanderthals with a higher risk of Covid-19 hospitalisation and severe complications. But as Covid-19 spreads around the world it is clear that lots of different populations are being severely affected, many of which do not have any Neanderthal genes.
“We must avoid simplifying the causes and impact of Covid-19, as ultimately a person’s response to the disease is about contact and then the body’s immunity response, which is influenced by many environmental, health and genetic factors.
Original article in The Guardian, Sept. 30, 2020
https://www.nature.com/articles/s41586-020-2818-3
Neanderthal DNA highlights complexity of COVID risk factors
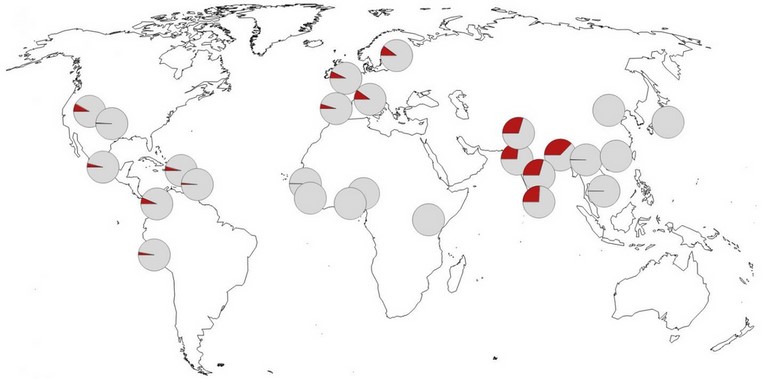
This figure appeared in the publication in Nature.These genetic variants are almost completely absent in Africa and occur in the highest frequency in Bangladesh. Professor Svante Pääbo and Professor Hugo Zeberg.
A genetic analysis reveals that some people who have severe reactions to the SARS-CoV-2 virus inherited certain sections of their DNA from Neanderthals. However, our ancestors can’t take all the blame for how someone responds to the virus.
A key part of tackling COVID-19 is understanding why some people experience more-severe symptoms than do others. Earlier this year, a segment of DNA 50,000 nucleotides long (corresponding to 0.002% of the human genome) was found to have a strong association with severe COVID-19 infection and hospitalization. Writing in Nature, Zeberg and Pääbo report that this region is inherited from Neanderthals. Their results not only shed light on one reason that some people are more susceptible to severe disease, but also provide insights into human evolutionary biology.
DNA sequences that are physically close to one other in the genome are often inherited (linked) together. These blocks of DNA, known as haplotypes, therefore contain tightly linked variants — DNA sequences or nucleotides that vary between individuals in a population. For example, the COVID-19 risk haplotype described earlier this year harbours variants across its entire 50,000-nucleotide span that are inherited together more than 98% of the time. Long haplotypes such as this could be a result of positive selection, maintained in our genomes because they contributed to our species’ chances of survival and reproductive success. They could also be introduced as a result of interbreeding with archaic hominin species such as the Denisovans and Neanderthals.
Some 1–4% of the modern human genome comes from these ancient relatives. Many of the surviving archaic genes are harmful to modern humans, and are associated with infertility and an increased risk of disease. But a few are beneficial. Examples include the Denisovan-like version of a gene called EPAS1 that helps modern Tibetans to cope with life at extremely high altitudes, a Neanderthal gene that increases our sensitivity to pain and others that help us fend off viruses.
To investigate whether the COVID-19 risk haplotype might have been introduced from our ancient relatives, Zeberg and Pääbo compared the region with an online database of archaic genomes from around the world. They found the region to be closely related to that in the genome of a Neanderthal individual that lived in modern-day Croatia around 50,000 years ago, but it was not related to any known Denisovan genomes.
The authors next checked the prevalence of the Neanderthal-derived haplotype in the modern human population. They report that it is rare or completely absent in east Asians and Africans. Among Latin Americans and Europeans, the risk haplotype is maintained at a modest frequency (4% and 8%, respectively). By contrast, the haplotype occurs at a frequency of 30% in individuals who have south Asian ancestry, reaching as high as 37% in those with Bangladeshi heritage (Fig. above).
The researchers therefore speculate that the Neanderthal-derived haplotype is a substantial contributor to COVID-19 risk in specific groups. Their hypothesis is supported by hospital data from the Office for National Statistics in the United Kingdom, which indicates that individuals of Bangladeshi origin in the country are twice as likely to die from COVID-19 as are members of the general population (although other risk factors will, of course, contribute to these statistics).
Why has this haplotype been retained in some populations? The authors posit that it might be protective against other ancient pathogens, and therefore positively selected for in certain populations around the world. But when individuals are infected with the SARS-CoV-2 coronavirus, the protective immune response mediated by these ancient genes might be overly aggressive, leading to the potentially fatal immune response observed in people who develop severe COVID-19 symptoms. As a result, a haplotype that at times in our past might have been beneficial for survival could now be having an adverse effect.
Despite the correlation between this risk haplotype and clinical outcomes, genetics alone do not determine a person’s risk of developing severe COVID-19. Our genes and their origins clearly influence the development and progression of COVID-19 (and other infectious diseases), but environmental factors also have key roles in disease outcomes.
For example, although the Neanderthal-derived risk haplotype is almost completely absent in people with African ancestry, this population has a higher COVID-19 mortality rate than do people of other ethnic backgrounds, even after adjusting for geography and socio-economic factors (see go.nature.com/3jcxezx (‘Demographics’ tab) and go.nature.com/2h4qfqu, for example). Social inequality and its repercussions seem likely to account for a larger proportion of the risk of COVID-19 death than does Neanderthal-derived DNA.
It is fascinating to think that our ancestor’s genetic legacy might be playing a part in the current pandemic. However, the underlying impact of the inherited DNA on the body’s response to the virus is unclear. Ongoing global efforts to study associations between our genetics and COVID-19 by analysing more individuals from diverse populations, such as that being undertaken by the COVID-19 Host Genetics Initiative (www.covid19hg.org), will help us to develop a better understanding of the disease’s aetiology. It is important to acknowledge that, although genes involved in the COVID-19 response might be inherited, social factors and behaviours (such as social distancing and mask wearing) are in our control, and can effectively reduce the risk of infection.
Yang Luo in Nature 587, 552-553 (2020)doi: https://doi.org/10.1038/d41586-020-02957-3
Go to the original article in Nature.
Neanderthals may have had heightened sensitivity to pain

Neanderthals may have experienced more pain than average modern humans do, according to new research led by scientists from the Max Planck Institute for Evolutionary Anthropology, Karolinska Institutet and Okinawa Institute of Science and Technology.
Neanderthals and their Asian relatives, Denisovans, evolved separately from the ancestors of present-day humans for about 500,000 years. During that time, each group independently accumulated genetic changes that became frequent or fixed. However, late in their history, Neanderthals and Denisovans mixed with modern humans, which resulted in many genetic variants from these ancient hominins being present in people today.
As several Neanderthal genomes of high quality are now available, it is possible to identify genetic changes that occurred in Neanderthals, investigate their physiological effects, and assess their consequences when they occur in humans today.
One such case is the gene SCN9A, which encodes the Nav1.7 protein, a sodium channel crucial for impulse generation and conduction in peripheral pain pathways.
“Pain is mediated through specialized nerve cells that are activated when potentially harmful things affect various parts of our bodies,” said lead author Dr. Hugo Zeberg, a researcher at the Max Planck Institute for Evolutionary Anthropology and Karolinska Institutet. “These nerve cells have a special ion channel that has a key role in starting the electrical impulse that signals pain and is sent to the brain.”
Dr. Zeberg and colleagues analyzed 2,535 human genomes from in the 1000 Genomes (1000G) Project and found that some present-day people from Europe and Central and South America carry a Neanderthal variant of the sodium channel Nav1.7. They also found that carriers of this Neanderthal variant experience more pain.
“The biggest factor for how much pain people report is their age,” Dr. Zeberg said. “But carrying the Neanderthal variant of the ion channel makes you experience more pain similar to if you were eight years older.”
“The Neanderthal variant of the ion channel carries three amino acid differences — M932L, V991L and D1908G — to the common, modern variant.” “While single amino acid substitutions do not affect the function of the ion channel, the full Neanderthal variant carrying three amino acid substitutions leads to heightened pain sensitivity in present-day people.”
On a molecular level, the Neanderthal Nav1.7 ion channel is more easily activated which may explain why people who inherited it have a lowered pain threshold.
“Whether Neanderthals experienced more pain is difficult to say because pain is also modulated both in the spinal cord and in the brain,” said senior author Dr. Svante Pääbo, a scientist at the Max Planck Institute for Evolutionary Anthropology and Okinawa Institute of Science and Technology. “But this work shows that their threshold for initiating pain impulses was lower than in most present-day humans.”
The research is published in the journal Current Biology. Hugo Zeberg et al. 2020. A Neanderthal Sodium Channel Increases Pain Sensitivity in Present-Day Humans. Current Biology 30: 1-5; doi: 10.1016/j.cub.2020.06.045
Enrico de Lazaro
Original article in sci-news.com
Neanderthals were as good at tolerating smoke-related toxins as Early modern humans: study
A new genetic study conducted by researchers from Leiden University and Wageningen University thoroughly debunks previous claims that a genetic mutation gave early Homo sapiens an evolutionary advantage over Neanderthals in adapting to campfire smoke exposure.
“Making and using fire is regarded as one of the most significant innovations in man’s evolution,” said Dr. Jac Aarts, lead author on the current study, and colleagues.“Fire brought with it such benefits as warmth, for example, protection against predators and a broader diet because it made it possible to cook raw, inedible foods.” “A disadvantage of fire is that it exposes people to the toxic substances in smoke,” they added.
In 2016, Pennsylvania State University researchers looked at the aryl hydrocarbon receptor (AHR) gene — which regulates the human body’s response to carcinogenic polycyclic aromatic hydrocarbons produced by fires — in modern humans and Neanderthals.
They found that modern humans carry a mutation in the AHR gene that increased their tolerance to smoke-related toxins.
They concluded that Neanderthals were up to 1,000 times more sensitive to these toxins than modern humans.
In 2018, Dr. Aarts’ team came to the opposite conclusion, based on an analysis of 19 relevant genes in Neanderthal, Denisovan, prehistoric and extant anatomically modern human genomes.
They found that Neanderthals had more gene variants that better neutralized the harmful effects of toxins than most modern humans.
In the new study, Dr. Aarts and co-authors repeated the earlier experiments of their colleagues from Pennsylvania.
Instead of rat cells, the researchers used human cells and found that there are no grounds for concluding that the AHR protein made Neanderthals more vulnerable to toxins in the smoke.
“Our results are strongly at odds with a major role of the modern human AHR in the evolution of hominin detoxification of smoke components and consistent with our previous study based on 18 relevant genes in addition to AHR, which concluded that efficient detoxification alleles are more dominant in ancient hominins, chimpanzees and gorillas than in modern humans,” they said.
Their results were published in the journal Molecular Biology and Evolution.
_____
Jac M.M.J.G. Aarts et al. Evolution of Hominin Detoxification: Neanderthal and Modern Human Ah Receptor Respond Similarly to TCDD. Molecular Biology and Evolution, published online November 24, 2020; doi: 10.1093/molbev/msaa287
A new genetic study conducted by researchers from Leiden University and Wageningen University thoroughly debunks previous claims that a genetic mutation gave early Homo sapiens an evolutionary advantage over Neanderthals in adapting to campfire smoke exposure.
“Making and using fire is regarded as one of the most significant innovations in man’s evolution,” said Dr. Jac Aarts, lead author on the current study, and colleagues. “Fire brought with it such benefits as warmth, for example, protection against predators and a broader diet because it made it possible to cook raw, inedible foods.” “A disadvantage of fire is that it exposes people to the toxic substances in smoke,” they added.
In 2016, Pennsylvania State University researchers looked at the aryl hydrocarbon receptor (AHR) gene — which regulates the human body’s response to carcinogenic polycyclic aromatic hydrocarbons produced by fires — in modern humans and Neanderthals.
They found that modern humans carry a mutation in the AHR gene that increased their tolerance to smoke-related toxins. They concluded that Neanderthals were up to 1,000 times more sensitive to these toxins than modern humans.
In 2018, Dr. Aarts’ team came to the opposite conclusion, based on an analysis of 19 relevant genes in Neanderthal, Denisovan, prehistoric and extant anatomically modern human genomes. They found that Neanderthals had more gene variants that better neutralized the harmful effects of toxins than most modern humans.
In the new study, Dr. Aarts and co-authors repeated the earlier experiments of their colleagues from Pennsylvania.
Instead of rat cells, the researchers used human cells and found that there are no grounds for concluding that the AHR protein made Neanderthals more vulnerable to toxins in the smoke.
“Our results are strongly at odds with a major role of the modern human AHR in the evolution of hominin detoxification of smoke components and consistent with our previous study based on 18 relevant genes in addition to AHR, which concluded that efficient detoxification alleles are more dominant in ancient hominins, chimpanzees and gorillas than in modern humans,” they said.
Their results were published in the journal Molecular Biology and Evolution. Jac M.M.J.G. Aarts et al. Evolution of Hominin Detoxification: Neanderthal and Modern Human Ah Receptor Respond Similarly to TCDD. Molecular Biology and Evolution, published online November 24, 2020; doi: 10.1093/molbev/msaa287
Enrico de Lazaro, original article in sci-news.com
Neanderthal thumbs were better adapted to holding tools with handles: study
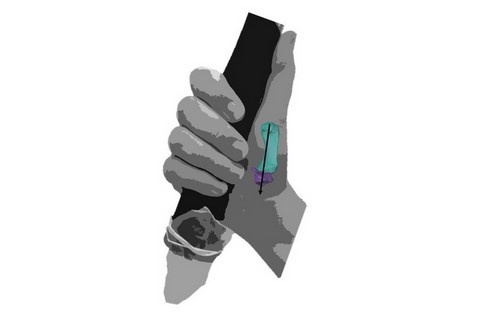
Neanderthals may have found precision grips (where objects are held between the tip of the finger and thumb) more challenging than power squeeze grips (where objects are held like a hammer, between the fingers and the palm with the thumb directing force), according to new research led by the University of Kent.
"Much research has debated the technological abilities of Neanderthals (Homo neanderthalensis) relative to those of early modern humans (Homo sapiens), with a particular focus on subtle differences in thumb morphology and how this may reflect differences in manipulative behaviors in these two species,” said lead author Dr. Ameline Bardo from the Skeletal Biology Research Centre at the University of Kent and colleagues.
“We provide a novel perspective on this debate through a 3D geometric morphometric analysis of shape covariation between the trapezial and proximal first metacarpal articular surfaces of Neanderthals in comparison to early and recent humans.”
The researchers used 3D analysis to map the joints between the bones responsible for movement of the thumb (referred to collectively as the trapeziometacarpal complex) of five Neanderthal individuals. They then compared the results to measurements taken from the remains of five early modern humans and 40 recent modern adults.
They found covariation in shape and relative orientation of the trapeziometacarpal complex joints that suggest different repetitive thumb movements in Neanderthals compared with modern humans.
The joint at the base of the thumb of the Neanderthal remains is flatter with a smaller contact surface, and better suited to an extended thumb positioned alongside the side of the hand. This thumb posture suggests the regular use of power ‘squeeze’ grips, like the ones we now use to hold tools with handles.
In comparison, these joint surfaces are generally larger and more curved in recent modern human thumbs, an advantage when gripping objects between the pads of the finger and thumb, known as a precision grip.
“Although the morphology of the studied Neanderthals is better suited for power ‘squeeze’ grips, they would still have been capable of precision hand postures — but they would have found this more challenging than modern humans,” Dr. Bardo said.
“Comparison of fossil morphology between the hands of Neanderthals and modern humans may provide further insight into the behaviours of our ancient relatives and early tool use.”
The team’s paper was published online in the journal Scientific Reports.
_____
A. Bardo et al. 2020. The implications of thumb movements for Neanderthal and modern human manipulation. Sci Rep 10, 19323; doi: 10.1038/s41598-020-75694-2
Oiginal article in sci-news.com

